Tobramycin data set
Download data set: tobramycin.txt
This data set has been originally published in:
Tobramycin is an antimicrobial agent of the aminoglycosides family, which is among others used against severe gram-negative infections. Because tobramycin does not pass the gastro-intestinal tract, it is usually administrated intravenously as intermittent bolus doses or short infusions. Tobramycin is a drug with a narrow therapeutic index.
Tobramycin bolus doses ranging from 20 to 140mg were administrated every 8 hours in 97 patients (45 females, 52 male) during 1 to 21 days (for most patients, during ~6 days). Age, weight (kg), sex and creatinine clearance (mL/min) were available as covariates. The tobramycin concentration (mg/L) was measured 1 to 9 times per patients (322 measures in total), most of the time between 2 and 6h post-dose. This sparse data set is presented on the figure below
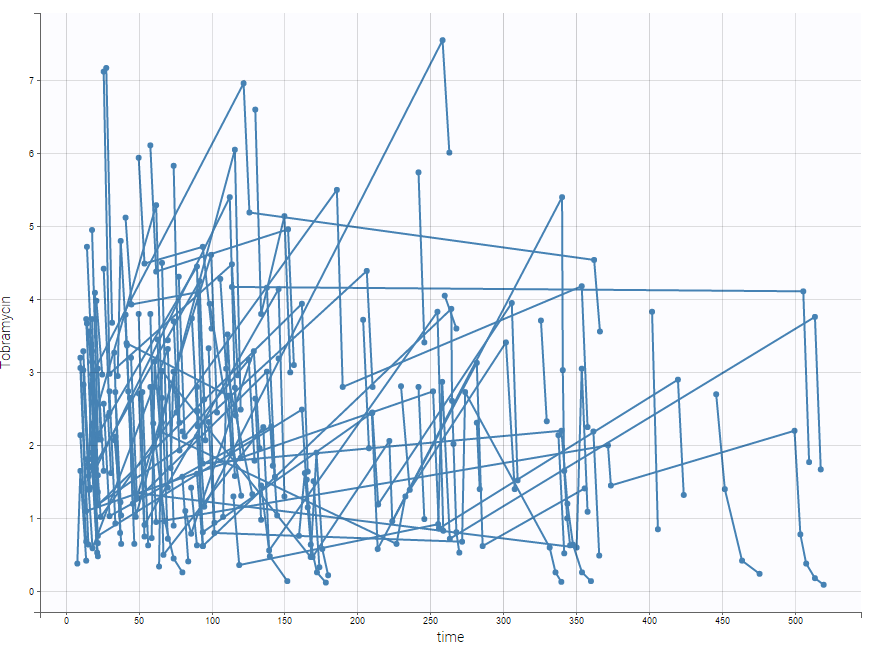
Below is an extract of the data set:
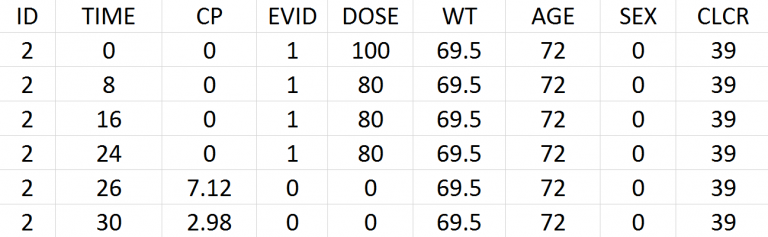
The columns have the following meaning:
ID: the subject’s ID, column-type ID.
TIME: the time of the record (administration or measurement) in hours, column-type TIME.
CP: the measured drug’s concentration in mg/L, column-type OBSERVATION.
EVID: event identifier, 1 for doses and 0 for response lines (measurements), column-type EVENT ID.
DOSE: the amount of drug administrated to this subject in mg, column-type AMOUNT.
WT: weight of the subject in kg, column-type CONTINUOUS COVARIATE.
SEX: sex of the subject, column-type CATEGORICAL COVARIATE.
CLCR: creatinine clearance of the subject in mL/min, column-type CONTINUOUS COVARIATE.
Several points can be noticed:
The four first lines correspond to doses, while the other ones are measurements, as indicated by the EVID column. The MDV column is not necessary. The zeros of the DOSE and CP columns could have been replaced by dots ‘.’ .
The covariates columns (WT, SEX and CLCR) are filled with the same value for each individual. Covariates must be constant within subjects (or subject-occasions when occasions are defined).
